
Delays, non-compliance and supplier failure risk more than timelines. They threaten regulatory inspections and capital.
Gatekeeper surfaces key dates, risks and deliverables across your entire biotech portfolio. Our LuminIQ AI agents monitor every supplier and contract milestone, so you can act before risk becomes disruption.
Stay audit-ready. Hit every deadline. Protect every investment.

-1.png?width=724&height=468&name=DocuSign-signature%20(1)-1.png)
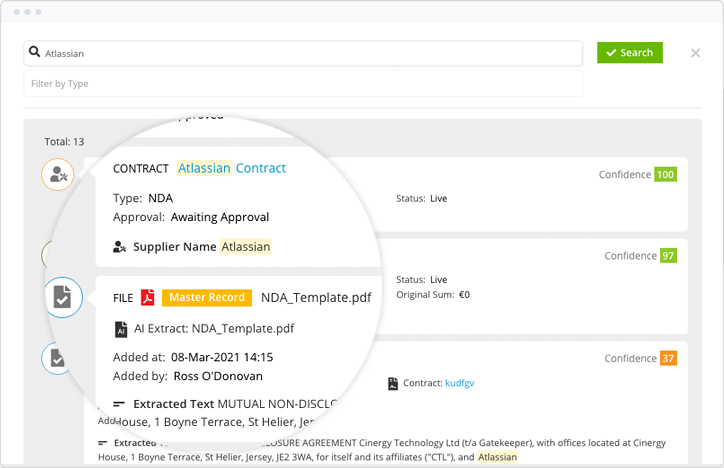
Manual contract approvals risk MHRA breaches, missed milestones and inspection delays.
Gatekeeper replaces inconsistent sign-off processes with AI-powered, best practice workflows. Tailored by value, risk and document type, nothing gets missed and every approver is accountable.
Create an automated audit trail from first draft to signature. Reduce turnaround times. Prove compliance at every step.
-Mar-27-2025-01-21-11-8144-PM.png)


-3.png)


High-stakes agreements, from licensing and IP rights to CRO contracts, demand both speed and regulatory certainty.
Gatekeeper supports Qualified Electronic Signatures (QES) to meet global standards like eIDAS. Every signature is fully traceable, tamper-proof, and audit-ready, accelerating contract approvals without compromising compliance.
Close faster, stay compliant, and safeguard your most valuable research assets.

Protect your clinical trials and IP by automating third-party compliance across your biotech portfolio.
Our AI Agents continuously surface risks, financial changes, and cybersecurity threats in your supply chain, before they threaten inspections or disrupt funding.
Monitor every contract and supplier agreement against FDA, EMA, REACH, and USPTO standards in one auditable, inspection-ready system.


Every missed renewal risks late-stage trial delays, stalled production, or IP exposure. With Gatekeeper, you stay ahead of every agreement across your CDMOs, CROs, and supply chain.
Our AI Agents surface upcoming renewals, helping you to renegotiate proactively and prevent costly disruptions before they happen.
Avoid penalties, renegotiate smarter, and protect continuity across your research operations.
.png)
.png)
.png)
-4.png)
Before Gatekeeper, our contracts
Anastasiia Sergeeva, Legal Operations Manager, BlaBlaCar
were everywhere and nowhere.
Gatekeeper is that friendly tap on the shoulder,
Donna Roccoforte, Paralegal, Hakkasan Group
to remind me what needs our attention.
Great System. Vetted over 25 other systems
Randall S. Wood, Associate Corporate Counsel, Cricut
and Gatekeeper rose to the top.
Thank you for requesting your demo.
Next Step - Book a Call
Please book a convenient time for a quick call to discuss your requirements.